Gut microbiome and Immunity
Our immune system is very complex and provides the vital function of protecting us from invaders/pathogens, such as viruses, bacteria, parasites, fungi, toxins, etc. A good analogy for the immune system is a bank – the bank has various mechanisms to protect all the valuables inside (our blood and organs) from robbers (the pathogens).
Our immune system uses two types of immunity to protect us – innate (non-specific) immunity and adaptive (specific) immunity.
Innate immunity is what we are born with and includes external and internal defences to keep out the robbers or to apprehend and eliminate them if they do get into the bank.
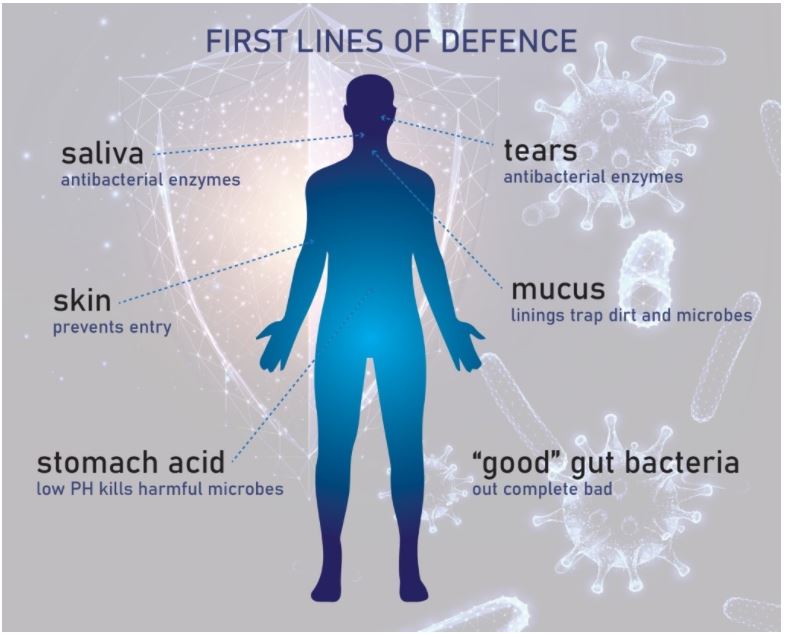
The external defences are represented by the bank’s walls – these are the physical and chemical barriers of the innate immune system, for example:
- Our skin and gut epithelium (digestive tract walls) – the main physical barriers that cover our body’s surfaces.
- Saliva and tears – these contain antibacterial enzymes to break down bacteria and can be used to flush/wash them out of our mouth and eyes.
- Mucus – this thick fluid lines our respiratory and digestive tracts and can trap dirt and microbes.
- Stomach acid – the low pH of the acid in our stomach can kill acid-intolerant microbes
The bank’s security guards represent the internal defences. These are the components of the inflammatory response including defence compounds, such as cytokines and defensins, and white blood cells, namely phagocytes (which include macrophages, dendritic cells and neutrophils) and natural killer cells. Phagocytes eat and destroy other cells by a process called phagocytosis – the phagocytes identify and respond to pathogens and infected/dead/damaged cells, bind to them, and then engulf and destroy them.
Our adaptive immunity is developed over time and is based on having exposure to things, like a disease/virus or a vaccine. If the robbers get past the bank walls and overwhelm the security guards, then the security guards (macrophages and dendritic cells) trigger the alarm system (they become antigen presenting cells (APCs)) to call for backup from the police – the lymphocytes, known as T and B cells.
The police can deploy additional weapons to fight off or capture the robbers. For example, T cells, through the cell-mediated response, are activated by the APCs and then differentiate into various types of T cells to help fight the infection – cytotoxic T cells directly attach to and kill pathogens and virus-infected cells, while helper T cells stimulate the response of B cells and macrophages.
APCs and T helper cells stimulate B cells, which produce antibodies to bind and neutralise pathogens. The antibodies are specific to a particular antigen and once bound to that antigen on a cell/pathogen,
they are effectively in handcuffs/jail – the cell/pathogen is no longer able to move, reproduce and infect other cells.
While this is going on, memory T and B cells (our CCTV capturing footage of the robbers) are produced and remain in the lymphatic tissue so that if the same robber returns they are able to form a stronger, faster response.
How does our gut microbiome fit with our immune system?
The human body actually contains about the same number of bacterial cells as human ones. Within and on our body are trillions of microbes, collectively known as microbiota, or when the genetic material of these microbes is included, this is the microbiome, however the two words tend to be used interchangeably these days.
Approximately 95% of the trillions of microbes we host, reside in our gut. This gut microbiome is of particular interest to us being of great importance for not only our intestinal health, but our immune, mental and general health and wellbeing (Martin, Bermudez-Humaran, & Langella, 2018).
The gut microbiome interacts with the human body and plays a vital role in:
- Normal gut development
- Promotion of fat storage
- Promotion of blood vessel formation
- Modulation of bone density
- Synthesis of vitamins and amino acids
- Modification of the nervous system (gut-brain axis)
- Breaking down food compounds
- The immune system.
The gut microbiome can affect and support the immune system in several ways.
Before we get into that, let’s set the scene. Our gastrointestinal tract has several key components: the gut epithelium (a layer of intestinal epithelial cells that form part of our bank’s walls), the intestinal lumen, and the lamina propria. Lining the epithelium is mucus and within the lumen are the beneficial microbes and pathogens. Within the lamina propria are the cells of our immune system, including dendritic cells and macrophages (those security guards).
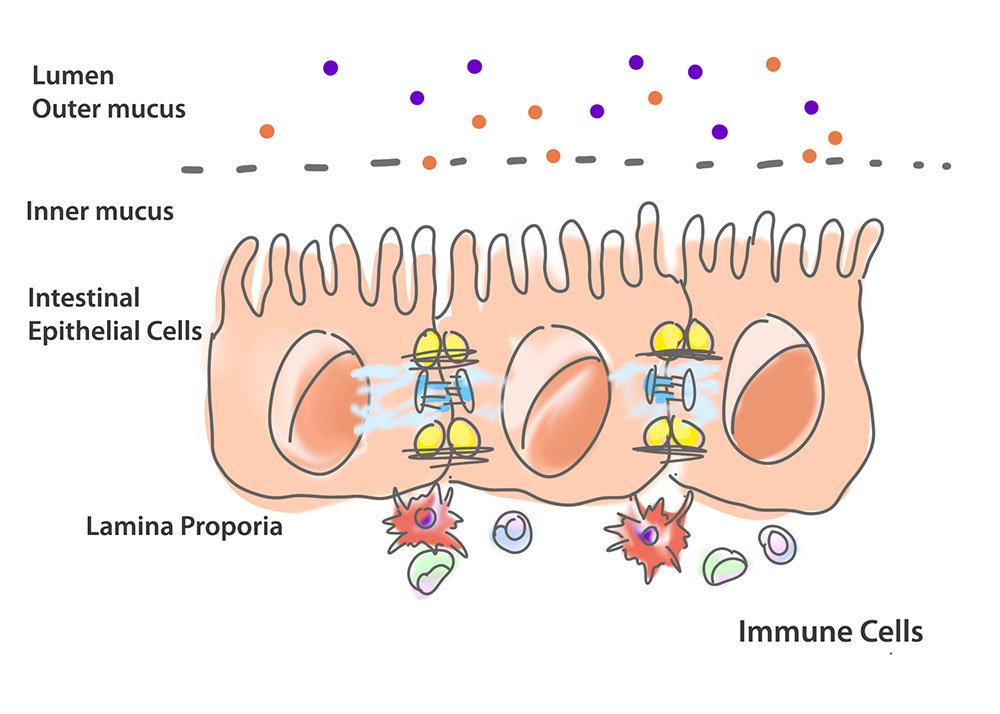
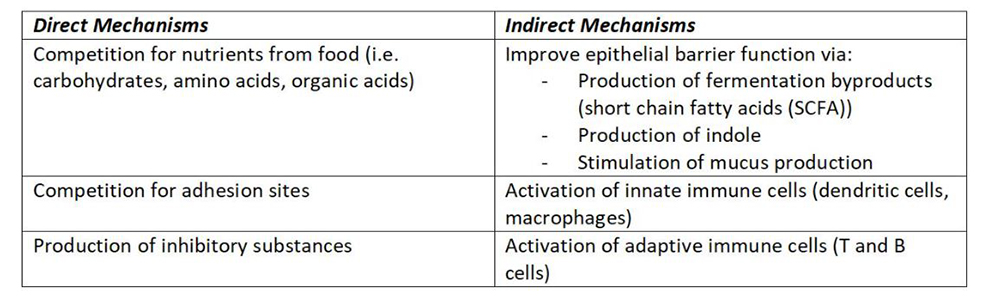
The beneficial bacteria provide resistance to pathogens (robbers). This can be via direct or indirect mechanisms (Kamada, Seo, Chen, & Nunez, 2013).
The beneficial microbes also help to modulate, develop and train the immune system (i.e. training of the security guards and police). Microbial colonisation of the gastrointestinal tract appears to start before birth, however, the largest share of colonisation occurs after birth with microbes mainly originating from the mother. The first 1000 days after birth are the most critical with delivery mode, breastfeeding, the introduction of solids, and environmental factors all playing a role in the development and diversification of the gut microbiome. The commensal microorganisms (resident, ‘good’ microbes)
interact with the host and help the immune system differentiate between them (they become like “self”) and the pathogenic bacteria through toll-like receptors (TLRs).
It’s a Two-Way Street
The interaction with the immune system is not just one-way and the condition of both the gut microbiome and the immune system affects that of the other. Unbalanced and poor gut microbiomes can lead to immune health issues such as autoimmunity, allergies and metabolic disorders, and a poor immune system can also cause poor and unbalanced microbiomes. For example, in inflammatory bowel disease (IBD), mutations in genes involved in the immune system can disrupt the gut microbiome, causing dysbiosis, and the loss of protective bacteria and/or the accumulation of pathogens (dysbiosis) leads to chronic inflammation (Kamada, Seo, Chen, & Nunez, 2013).
Likewise, a diverse, rich and balanced microbiome supports a normal immune system which in turn supports a diverse, rich and balanced microbiome. This is referred to as homeostasis, whereby the beneficial microbiota, such as the likes of Faecalibacterium prausnitzii, have anti-inflammatory effects and promote regulatory immune responses involving T regulatory cells, interleukin-10 (IL-10) and antimicrobial compounds, and the immune system, especially via IgA production, helps to promote a rich and balanced bacterial community by keeping pathogenic bacteria at bay.
How to keep your gut microbiome and immune system happy
In order to help sustain your gut microbiota and immune system, you should consume prebiotics. Prebiotics, in the form of dietary fibre, such as kiwifruit pectin, are food for good gut bacteria. The bacteria ferment the dietary fibre to generate energy allowing them to grow and produce essential short-chain fatty acids. More beneficial bacteria and their byproducts mean they are able to outcompete the pathogens and support the immune system.
Eating a range of complex prebiotics, like Livaux gold kiwifruit powder with its complex kiwi fruit pectin, allows for the growth of a range of beneficial gut bacteria resulting in a desirable rich, diverse and balanced gut microbiome and healthy immune system.
References
- Kamada, N., Seo, S., Chen, G., & Nunez, G. (2013). Role of the gut microbiota in immunity and inflammatory disease. Nature Reviews: Immunology, 13: 321-335.
- Martin, R., Bermudez-Humaran, L., & Langella, P. (2018). Searching for the bacterial effector: the example of the multi-skilled commensal bacterium Faecalibacterium prausnitzii. Frontiers in Microbiology, 9: 346.